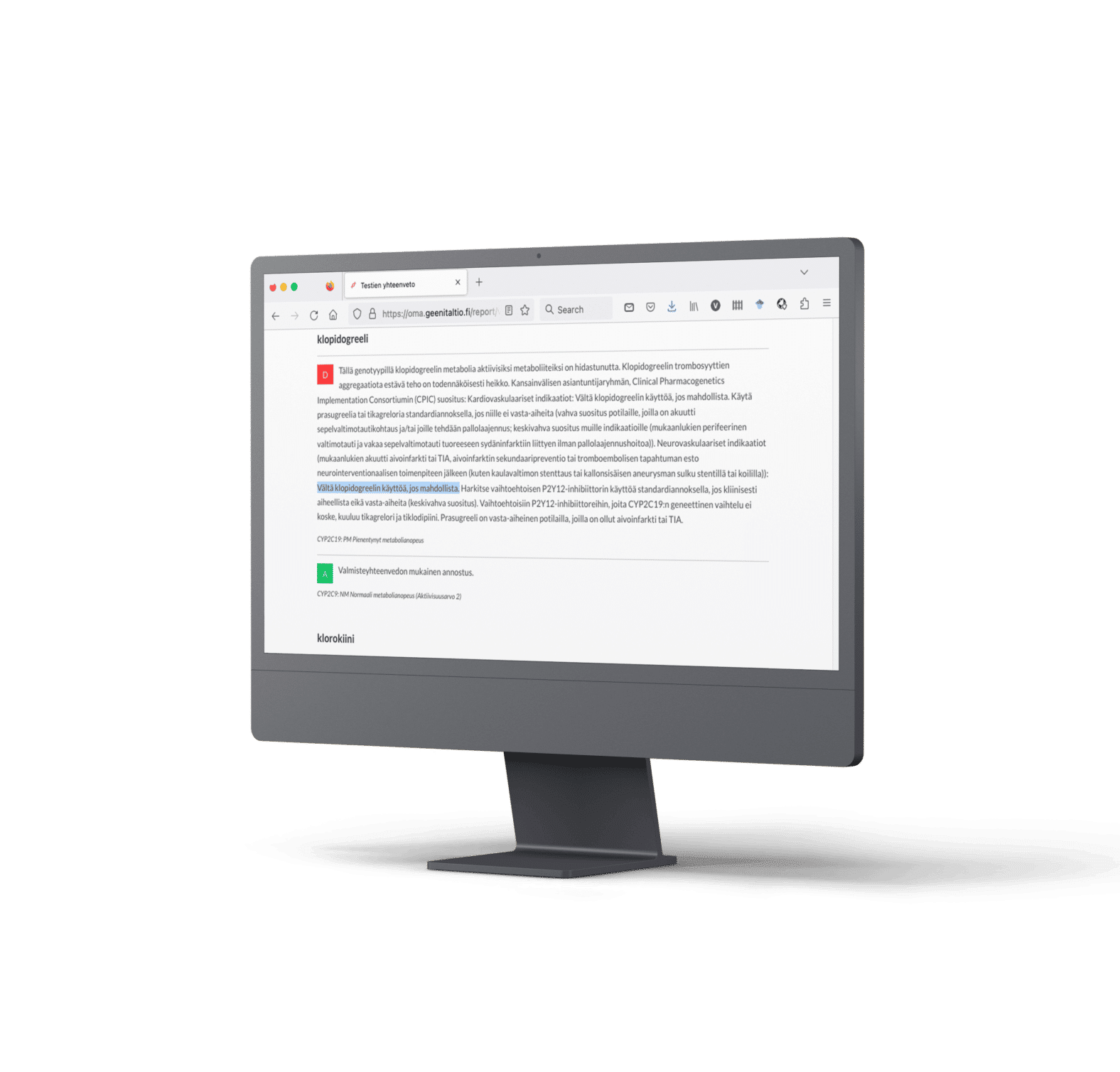
Clopidogrel is a prodrug metabolized via CYP2C19 to its active form. Individuals with slowed CYP2C19 metabolism may not convert the drug adequately to its active form, leading to inadequate efficacy in preventing blood clots. Up to a third of patients have a slower CYP2C19 metabolism 2, 6.
ESC advises against using clopidogrel in these patients with poor or intermediate CYP2C19 metabolism and recommends considering pharmacogenetic testing before initiating clopidogrel in patients at risk of thrombosis or bleeding. ESC emphasizes the importance of testing to prevent stent thrombosis. 2
The ESC guideline for ACS states that de-escalation from ticagrelor/prasugrel to clopidogrel guided by CYP2C19 genotyping in ACS patients undergoing PPCI within the previous 48 h is non-inferior to standard treatment with ticagrelor or prasugrel at 12 months with respect to thrombotic events and results in a lower incidence of bleeding.7, 8
In a meta-analysis evaluating the cost-effectiveness of pharmacogenetic testing, 22 out of 23 studies found testing to be cost-effective or cost-saving when prescribing clopidogrel.5 The total cost of treatment guided by pharmacogenetic testing is 50% less expensive than conventional therapy with clopidogrel, according to a Spanish study.9
Significant genetic variability is associated with the efficacy of medications used in the treatment of cardiovascular diseases, particularly clopidogrel. Pharmacogenetic testing is also beneficial for patients using warfarin, simvastatin, and metoprolol. According to a meta-analysis, pharmacogenetic testing reduces the risk of major adverse cardiovascular events (MACE) by 41%, bleeding risk by 25%, and risk of myocardial infarction by 47%, in patients with ACS or undergoing PCI. 4
Warfarin is significantly influenced by CYP2C9 and VKORC1 enzymes. ESC recommends pharmacogenetic testing before starting warfarin 2 to adjust the dosage according to the patient’s phenotypes.
Statin use, especially with simvastatin, pose a risk of myopathy significantly influenced by pharmacogenetics. According to ESC’s recommendation, high-dose simvastatin (80 mg) should be avoided, and alternative statin therapy should be considered if the patient is a carrier of the SLCO1B1*5 variant 2 2.
Metoprolol metabolism is highly dependent on CYP2D6, so ESC advises against metoprolol use if the test indicates the patient is a slow or ultrarapid CYP2D6 metabolizer 2.
[1] Häkkinen, K. et al. (2022) Implementation of CYP2D6 copy-number imputation panel and frequency of key pharmacogenetic variants in Finnish individuals with a psychotic disorder. The Pharmacogenomics Journal. 22, 166-172. https://doi.org/10.1038/s41397-022-00270-y
[2] Magavern, E. et al. (2022). The role of pharmacogenomics in contemporary cardiovascular therapy: a position statement from the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. European Heart Journal-Cardiovascular Pharmacotherapy, 8(1), 85-99. https://doi.org/10.1093/ehjcvp/pvab018
[3] Duodecim Terveysportti Lääketietokanta
[4] Kheiri, B. et al. (2020) Genotype-Guided Strategy for P2Y12 Inhibitors in Coronary Artery Disease: A Meta-Analysis of Randomized Clinical Trials. J Am Coll Cardiol Intv. 13 (5) 659–661. https://doi.org/10.1016/j.jcin.2019.11.019
[5] Morris, S.A., Alsaidi, A.T., Verbyla, A., Cruz, A., Macfarlane, C., Bauer, J. and Patel, J.N. (2022), Cost Effectiveness of Pharmacogenetic Testing for Drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines: A Systematic Review. Clin Pharmacol Ther. https://doi.org/10.1002/cpt.2754
[6] Scott, S., Sangkuhl, K., Gardner, E. E., Stein, C. M., Hulot, J. S., Johnson, J. A., … & Shuldiner, A. R. (2011). Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450–2C19 (CYP2C19) genotype and clopidogrel therapy. Clinical Pharmacology & Therapeutics, 90(2), 328-332. https://doi.org/10.1038/clpt.2011.132
[7] Byrne, R. A., Rossello, X., Coughlan, J. J., Barbato, E., Berry, C., Chieffo, A., … & Ibanez, B. (2023). 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). European heart journal, 44(38), 3720-3826. https://doi.org/10.1093/eurheartj/ehad191
[8] Claassens, D. M., Vos, G. J., Bergmeijer, T. O., Hermanides, R. S., Van’t Hof, A. W., Van Der Harst, P., … & Ten Berg, J. M. (2019). A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. New England Journal of Medicine, 381(17), 1621-1631. https://doi.org/10.1056/NEJMoa1907096
[9] Koufaki, M. I., Fragoulakis, V., Díaz-Villamarín, X., Karamperis, K., Vozikis, A., Swen, J. J., … & Mitropoulou, C. (2023). Economic evaluation of pharmacogenomic-guided antiplatelet treatment in Spanish patients suffering from acute coronary syndrome participating in the U-PGx PREPARE study. Human Genomics, 17(1), 1-15. https://doi.org/10.1186/s40246-023-00495-3
The amount of scientific data on pharmacogenetics is vast and continuously growing and it can be difficult to stay on top of all the emerging research along with your clinical work. This is why the Abomics pharmacogenetic report compiles all the pharmacogenetic knowledge relevant to the clinician as usage and dosing recommendations of medications. Our team of medical doctors with expertise in pharmacogenetics follow closely all the new research in pharmacogenetics and update the recommendations in the report accordingly so that you can always have recommendations based on the latest research at your hand – without the need to go through all the research papers yourself.
The medication recommendations are based on the synthesis of information gathered by our board of medical advisors. The sources include peer-reviewed scientific literature of high quality, FDA’s (U.S. Food and Drug Administration) list of pharmacogenetic biomarkers in drug labels, EMA (European Medicines Agency), CPIC (Clinical Pharmacogenetics Implementation Consortium), and DPWG (Dutch Pharmacogenetics Working Group). The medication decisions for each patient are up to the treating doctor. The medication decisions for each patient are up to the treating doctor.
The Abomics pharmacogenetic report has recommendations for over 100 medicines based on the patient’s phenotype. We have designed the report especially for e.g., psychiatrists and general practitioners and using it doesn’t require specialisation in genetics. The report is easy to use as a medication decision support tool even during a short doctor’s appointment. The report has a summary of medicines that are significantly affected by the patient’s phenotype and practical guidelines for how to use a medicine for each patient specifically. The digital report is searchable so you can find the patient-specific recommendations easily with the name of the pharmaceutical substance.
Contact us about the availability of the test.

Abomics, Abomics PGx, GeneRx, GeneAccount are registered or non-registered trademarks of Abomics Oy in various countries.